-40%
Lutronic Infini
$ 7128
- Description
- Size Guide
Description
Quality Pre-ownedMedical Device Alliance in Frisco, Texas, USA
is a Market Leader in Pre-Owned
Aesthetic Technology Since 2009.
* Reach Out to Us For Your Questions *
Guaranteed Equipment
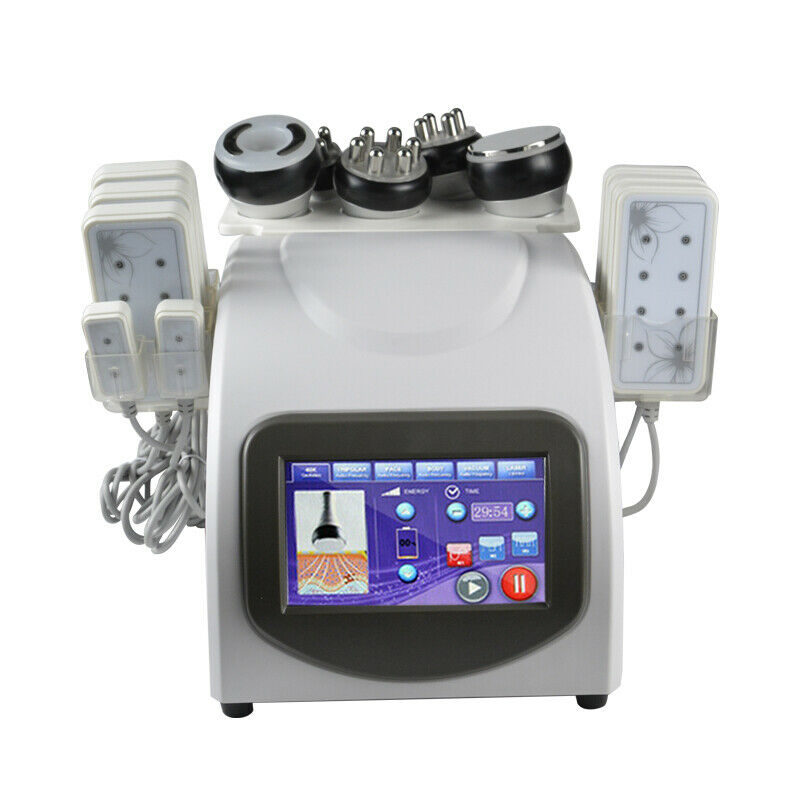
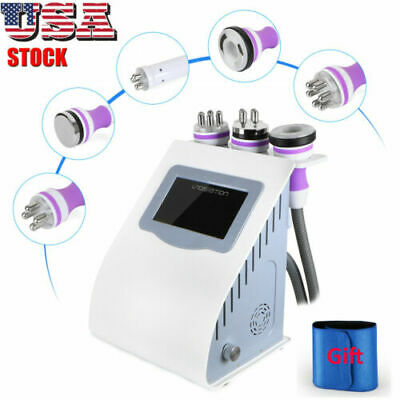
Lutronic Infini RF Microneedling- 2017
Infini Specifications
Date of Manufacture: 8/2017
Serial Number: R0617H003
System Shot Count 63,113
Treat Face, Submental, Neck, Periorbital
Infini Benefits and Applications
System Type:Bipolar RF (Radiofrequency)
Frequency: 1 MHz
Total RF Treatment Level: Up to Level 20 (Max. Power 50 W)
Operation Mode:Microneedle Fractional RF (MFR)
Treatment Duration:10 ms – 1,000 ms
Tips:MFR Microneedle electrodes, 0.5 – 3.5 mm (Adjustable depth) SFR: Up to 144 pin
Dual Switch:Foot & Finger Switch
Weight:28 kg
Dimension:362 mm (W) x 409 mm (L) x 1713 mm (H)
Pass: Pass1 : 2.25 – 3.5 mm, Pass2 : 1.5 – 2.25 mm, Pass3 : 1.0 – 1.25 mm
Intensity:Low, Medium, High
Infini Radiofrequency Microneedling
Payment
Shipping
Returns
Payment
Shipping
Returns
We accept payment by the following methods:
PayPal, Visa, Mastercard, with additional CC fee
ACH with no additional fee
Please pay within two days after winning an auction, as that will allow us to ship your item to you sooner!
All equipment requires a custom made, shock resistant packaging made to protect your device. Your order will be shipped within 10 business days (Monday-Friday) after cleared payment. You should expect to receive it two to five days after shipment
If item does not arrive as represented, y
ou can return a product for up to 14 days from the date you purchased it.
Any product you return must be in the same condition you received it and in the original packaging. Buyer will cover return shipping costs.
medical device alliance
medical device alliance
MEDICAL DEVICE ACKNOWLEDGEMENT:
By signing below, Buyer/Customer agrees to and understands the following:
Federal (USA) law restricts (and state law may restrict) this medical device to sale or use by or on the order of a physician, dentist, veterinarian or other practitioner licensed in the state in which this medical device is used or ordered (a "Prescribing Practitioner"). Customer and Customer's Prescribing Practitioner are solely responsible for the use and operation of this medical device in accordance with all applicable laws and regulations, and medical and treatment guidelines, and for ensuring that each operator of this medical device is adequately trained and qualified to use and operate this medical device safely and properly in a clinical setting and to perform medical procedures in accordance with such laws, regulations and guidelines. Medical Device Alliance makes no representations or warranties regarding federal, state or local laws or regulations, or medical or treatment guidelines, that might apply to the use and operation of this or any medical device. Customer and Customer's Prescribing Practitioner are solely responsible for contacting state and local licensing agencies regarding requirements applicable to the use and operation of this medical device. Use of this medical device involves certain risks of injury to patients. Customer and Customer's Prescribing Practitioner are solely responsible for ensuring that patients are informed of these risks. Improper use of this medical device may increase the risk of injury to patients.
Infini Specifications ---Date of Manufacture: 8/2017 ---Serial Number: R0617H003 ---System Shot Count 63,113 ---Treat Face, Submental, Neck, Periorbital Infini Benefits and Applications --- System Type:Bipolar RF (Radiofrequency) --- Frequency: 1 MHz --- Total RF Treatment Level: Up to Level 20 (Max. Power 50 W) --- Operation Mode:Microneedle Fractional RF (MFR) --- Treatment Duration:10 ms – 1,000 ms --- Tips:MFR Microneedle electrodes, 0.5 – 3.5 mm (Adjustable depth) SFR: Up to 144 pin --- Dual Switch:Foot & Finger Switch --- Weight:28 kg --- Dimension:362 mm (W) x 409 mm (L) x 1713 mm (H) ---Pass: Pass1 : 2.25 – 3.5 mm, Pass2 : 1.5 – 2.25 mm, Pass3 : 1.0 – 1.25 mm ---Intensity:Low, Medium, High Infini Radiofrequency Microneedling
Quality Pre-owned
Guaranteed Equipment
Medical Device Alliance in Frisco, Texas, USA
is a Market Leader in Pre-Owned
Aesthetic Technology Since 2009.
* Reach Out to Us For Your Questions *
Lutronic Infini RF Microneedling- 2017
Infini Specifications
Date of Manufacture: 8/2017
Serial Number: R0617H003
System Shot Count 63,113
Treat Face, Submental, Neck, Periorbital
Infini Benefits and Applications
System Type:Bipolar RF (Radiofrequency)
Frequency: 1 MHz
Total RF Treatment Level: Up to Level 20 (Max. Power 50 W)
Operation Mode:Microneedle Fractional RF (MFR)
Treatment Duration:10 ms – 1,000 ms
Tips:MFR Microneedle electrodes, 0.5 – 3.5 mm (Adjustable depth) SFR: Up to 144 pin
Dual Switch:Foot & Finger Switch
Weight:28 kg
Dimension:362 mm (W) x 409 mm (L) x 1713 mm (H)
Pass: Pass1 : 2.25 – 3.5 mm, Pass2 : 1.5 – 2.25 mm, Pass3 : 1.0 – 1.25 mm
Intensity:Low, Medium, High
Infini Radiofrequency Microneedling
medical device alliance
medical device alliance
Payment
We accept payment by the following methods:
PayPal, Visa, Mastercard, with additional CC fee
ACH with no additional fee
Please pay within two days after winning an auction, as that will allow us to ship your item to you sooner!
Shipping
All equipment requires a custom made, shock resistant packaging made to protect your device. Your order will be shipped within 10 business days (Monday-Friday) after cleared payment. You should expect to receive it two to five days after shipment
Returns
If item does not arrive as represented, y
ou can return a product for up to 14 days from the date you purchased it.
Any product you return must be in the same condition you received it and in the original packaging. Buyer will cover return shipping costs.
MEDICAL DEVICE ACKNOWLEDGEMENT:
By signing below, Buyer/Customer agrees to and understands the following:
Federal (USA) law restricts (and state law may restrict) this medical device to sale or use by or on the order of a physician, dentist, veterinarian or other practitioner licensed in the state in which this medical device is used or ordered (a "Prescribing Practitioner"). Customer and Customer's Prescribing Practitioner are solely responsible for the use and operation of this medical device in accordance with all applicable laws and regulations, and medical and treatment guidelines, and for ensuring that each operator of this medical device is adequately trained and qualified to use and operate this medical device safely and properly in a clinical setting and to perform medical procedures in accordance with such laws, regulations and guidelines. Medical Device Alliance makes no representations or warranties regarding federal, state or local laws or regulations, or medical or treatment guidelines, that might apply to the use and operation of this or any medical device. Customer and Customer's Prescribing Practitioner are solely responsible for contacting state and local licensing agencies regarding requirements applicable to the use and operation of this medical device. Use of this medical device involves certain risks of injury to patients. Customer and Customer's Prescribing Practitioner are solely responsible for ensuring that patients are informed of these risks. Improper use of this medical device may increase the risk of injury to patients.
Infini Specifications
Date of Manufacture: 8/2017
Serial Number: R0617H003
System Shot Count 63,113
Treat Face, Submental, Neck, Periorbital
Infini Benefits and Applications
System Type:Bipolar RF (Radiofrequency)
Frequency: 1 MHz
Total RF Treatment Level: Up to Level 20 (Max. Power 50 W)
Operation Mode:Microneedle Fractional RF (MFR)
Treatment Duration:10 ms – 1,000 ms
Tips:MFR Microneedle electrodes, 0.5 – 3.5 mm (Adjustable depth) SFR: Up to 144 pin
Dual Switch:Foot & Finger Switch
Weight:28 kg
Dimension:362 mm (W) x 409 mm (L) x 1713 mm (H)
Pass: Pass1 : 2.25 – 3.5 mm, Pass2 : 1.5 – 2.25 mm, Pass3 : 1.0 – 1.25 mm
Intensity:Low, Medium, High
Infini Radiofrequency Microneedling